SARAF Webinar, March 1st, 2022
Since 2001, SARAF aims at training scientists, engineers and technicians to the modern analytical methods dedicated to residues and contaminants in food.
A webinar offering an insight into new EU Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results has been broadcasted live, free of charge, on March 1st, 2022, from 9:00 to 12:00 CET.
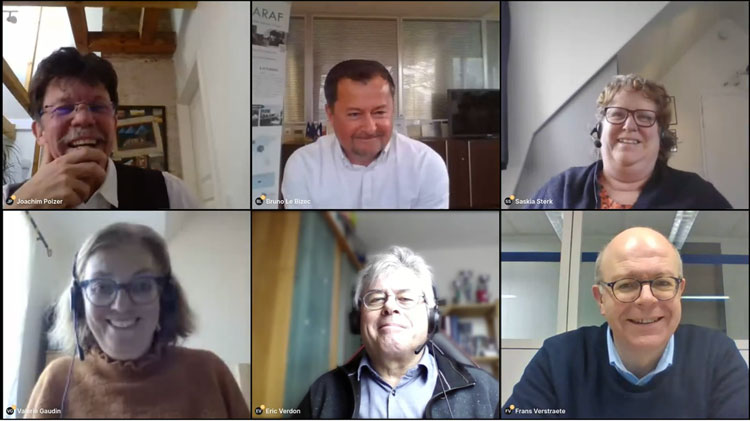
The presentations were given by key experts from 3 European Reference Laboratories (EURLs) for residues of veterinary medicines and contaminants in food of animal origin, according to the defined programme. The video as well as the pdf files are freely available. Many questions were posted by participants, to which detailed answers from the experts can be found below. Considering the audience and the live comments, the session was a great success.
Key experts
Bruno LE BIZEC
Moderator, NRL, LABERCA, Nantes, France
Frans VERSTRAETE
EU DG SANTE, Brussels, Belgium
Joachim POLZER
EURL Berlin, BVL, Germany
Eric VERDON
EURL Fougères, Anses, France
Valérie GAUDIN
EURL Fougères, Anses, France
Saskia STERK
EURL Wageningen, WFSR, The Netherlands
Programme
| The time schedule corresponds to the realised live: watch the replay | ||||
|---|---|---|---|---|
| 09:00 – 09:15(0:00:00) | INTRODUCTION | Presentation of the speakers, welcome to the participants Pr Bruno Le Bizec, NRL, LABERCA, Nantes, France |
||
| 09:15 – 09:45(0:09:55) | PRESENTATION | Current activities of the European Commission in the area of residues of veterinary medicines and the implementation of Reg. (EU) 2017/625
Mr Frans Verstraete, EU DG SANTE, Brussels, Belgium |
||
| 09:45 – 10:15(0:49:56) | PRESENTATION | Commission Implementing Regulation (EU) 2021/808 – Overview of changes
Dr Joachim Polzer, EURL Berlin, BVL, Germany |
||
| 10:15 – 10:25(1:26:20) | BREAK | |||
| 10:25 – 10:50(1:33:32) | PRESENTATION | EURL Guidance Document on Screening Method Validation (focus on microbiological, immunological and physico-chemical screenings)
Dr Eric Verdon and Dr Valerie Gaudin, EURL Fougères, Anses, France |
||
| 10:50 – 11:15(2:04:26) | PRESENTATION | EURL Guidance Document on Confirmation Method Validation (conventional approach)
Drs Saskia Sterk, EURL Wageningen, WFSR, The Netherlands |
||
| 11:15 – 11:40(2:26:46) | PRESENTATION | EURL Guidance Document on Confirmation Method Validation (alternative approach) Dr Joachim Polzer, EURL Berlin, BVL, Germany |
||
| 11:40 – 12:00(2:49:25) | CONCLUSION | 1-2 cross-cutting questions answered by each expert in his/her field | ||
Replay
Should you wish to download the video file (825 Mo), please contact us saraf(at)oniris-nantes.fr